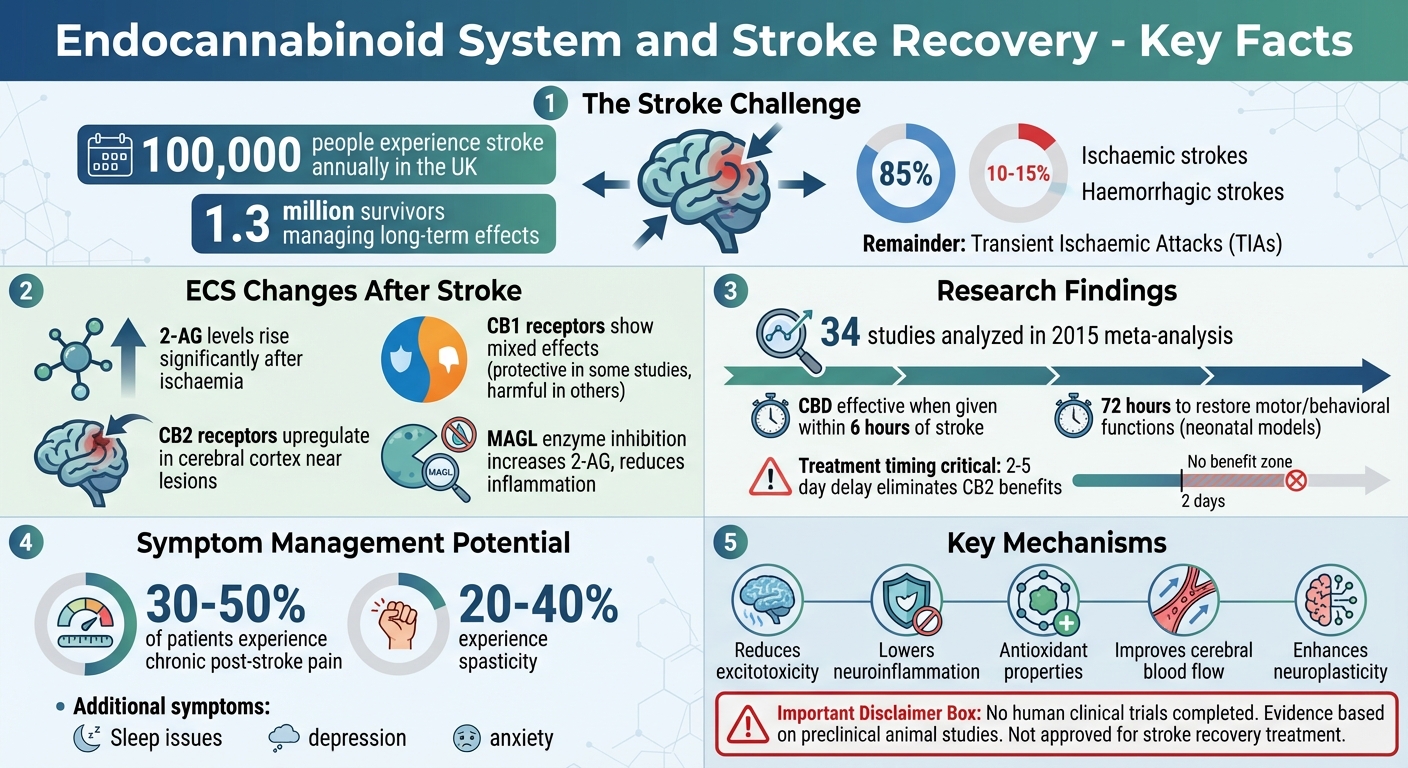
Every year, about 100,000 people in the UK experience a stroke, with 1.3 million survivors managing long-term effects. Recovery is challenging because strokes damage brain tissue, disrupt neural pathways, and trigger inflammation. However, the endocannabinoid system (ECS) – a network of receptors, enzymes, and cannabinoids in the body – may play a role in aiding recovery. Research suggests the ECS can help regulate inflammation, protect neurons, and support brain repair.
Key Takeaways:
- Stroke Types: Ischaemic (85% of cases), haemorrhagic (10–15%), and transient ischaemic attacks (TIAs).
- ECS Role: Helps manage brain inflammation, excitotoxicity, and blood flow after a stroke.
- Cannabinoids in Recovery: Preclinical studies show cannabinoids like CBD and synthetic compounds may reduce brain damage and improve functional outcomes.
- Challenges: Timing of treatment, mixed results in animal studies, and lack of human clinical trials.
Medical cannabis may help manage post-stroke symptoms like pain, spasticity, and mood disorders, but it’s not currently approved for stroke recovery. In the UK, specialist clinics like Elios Clinics offer tailored cannabis treatments under strict regulations.
While promising, ECS-based therapies remain experimental. More human studies are needed to confirm their effectiveness in stroke recovery.
Endocannabinoid System Role in Stroke Recovery: Key Statistics and Mechanisms
Cannabinoids & Strokes
How Stroke Affects the Endocannabinoid System
A stroke sets off rapid changes in the endocannabinoid system (ECS), triggering natural protective mechanisms in the brain. Understanding these shifts is key to why the ECS is increasingly being studied as a potential target for stroke therapies.
ECS Changes During and After Stroke
One of the most notable changes involves 2-arachidonoylglycerol (2-AG), the brain’s most abundant endocannabinoid. In rodent studies, 2-AG levels rise significantly after global ischaemia, linking endocannabinoid activity to the brain’s response to oxygen deprivation. This increase is especially pronounced in the peri-infarct area, where elevated 2-AG reduces the release of pro-inflammatory cytokines.
CB2 receptors also play a critical role in stroke response. Research using imaging techniques in rat stroke models has shown that CB2 receptors become more active in the cerebral cortex near ischaemic lesions. This upregulation appears to regulate inflammation in the affected tissue. Interestingly, when CB2 receptor agonists are given as a pretreatment in rat models of middle cerebral artery occlusion, they reduce neurodegeneration through anti-inflammatory effects. However, this benefit is lost if treatment is delayed by 2–5 days after the stroke, underlining the importance of timing.
The role of CB1 receptors is more complex and varies across studies. In some mouse models, activating CB1 receptors after a stroke protects neurons, astrocytes, and dendrites, while also improving motor function. Yet, other studies suggest that blocking CB1 receptors – or using mice without CB1 and CB2 receptors – reduces infarct size, inflammation, and leukocyte infiltration, while improving blood flow. These mixed results indicate that the effects of CB1 modulation depend on factors like stroke type and timing.
Enzymes that regulate endocannabinoid levels also become significant. For instance, monoacylglycerol lipase (MAGL), which breaks down 2-AG, emerges as a promising therapeutic target. In stroke models, using MAGL inhibitors like JZL184 and KML29 after stroke onset raises 2-AG levels, reduces arachidonic acid and inflammatory eicosanoids, and leads to smaller infarcts and better recovery outcomes. These findings highlight the potential for targeted ECS interventions in stroke recovery.
How ECS Modulation May Support Post-Stroke Recovery
Research suggests that modulating the ECS could help mitigate the damaging effects of a stroke. One way is by addressing excitotoxicity, a harmful process where excessive neurotransmitter activity overstimulates brain cells. CB1 receptors can help by reducing calcium influx into neurons, preventing toxic build-up and cell death.
ECS modulation also appears to reduce neuroinflammation, a key secondary process after a stroke. Elevated 2-AG levels and CB2 receptor activation work together to lower pro-inflammatory cytokines, reactive oxygen species, and cerebral vasoconstriction. In rat models, CB2 receptor agonists and MAGL inhibitors like JZL184 have been shown to limit inflammatory cell infiltration and reduce harmful eicosanoid production, creating a more supportive environment for neuron survival.
Additionally, the ECS may enhance neuroplasticity, the brain’s ability to form new connections and adapt to damage. Cannabinoids appear to promote neuroplasticity either through receptor pathways or by interacting with transcription factors, leading to improved recovery in preclinical studies. A 2015 meta-analysis of 34 studies confirmed that cannabinoids – whether derived from cannabis, synthetically produced, or targeting specific receptors – reduced infarct size and improved functional outcomes when administered after a stroke.
Despite these promising findings, challenges remain. For example, studies with CB1/CB2 receptor knockout mice suggest that agonist activity may sometimes worsen post-stroke outcomes. Additionally, the benefits of CB2 receptor agonists are only seen when treatment occurs during the acute phase. While preclinical data consistently show reductions in infarct size and improved recovery, human trials are still needed to confirm these effects in stroke patients.
Cannabinoids and Their Role in Stroke Recovery
Cannabinoids and Stroke Pathophysiology
Research on cannabinoids has shown encouraging results in preclinical studies, where they were found to reduce infarct size and improve recovery outcomes following a stroke. A meta-analysis highlighted that various types of cannabinoids – whether derived from plants, synthetically produced, or targeted as CB1 and CB2 receptor agonists – offered these benefits in both transient and permanent middle cerebral artery occlusion models.
For instance, CBD demonstrated its effectiveness in rodent models by reducing infarct size when administered within about six hours of stroke onset. In neonatal stroke models, a single dose of CBD immediately after the injury reduced neuronal damage, brain swelling, seizures, and blood flow issues. Remarkably, it also restored motor and behavioural functions within 72 hours. Another example is the synthetic cannabinoid WIN55,212-2, which, when given for seven days after injury in rodent models, supported long-term recovery of neurons and oligodendrocytes.
MAGL inhibitors, which work by increasing levels of the endocannabinoid 2-AG, have also shown promise. JZL184, for example, significantly reduced infarct size and improved recovery in both transient and permanent stroke models when administered post-stroke. Similarly, CPD-4645, another MAGL inhibitor, helped restore vascular balance and influenced gene expression in brain vessels in a photothrombotic stroke model.
Mechanisms Relevant to Stroke Recovery
Cannabinoids offer neuroprotection through a variety of interconnected mechanisms, as evidenced by preclinical models. CB1 receptors play a role in reducing excitotoxicity by blocking voltage-sensitive calcium channels, which prevents harmful calcium influx and subsequent cell death. Meanwhile, CB2 receptor activation and increased 2-AG signalling help reduce inflammation by lowering pro-inflammatory cytokines, decreasing leukocyte adhesion, and modulating microglial activity. This limits the secondary brain damage caused by inflammation.
Additionally, cannabinoids exhibit antioxidant properties. By suppressing reactive oxygen species and altering arachidonic acid-derived eicosanoid pathways – especially via MAGL inhibition – they help protect lipids, proteins, and DNA from oxidative damage. A 2023 review also pointed out that cannabinoids can improve astrocyte energy metabolism and shield glial cells from harmful stimuli, mechanisms that are particularly relevant to stroke recovery.
Beyond these effects, cannabinoids have been linked to improved cerebral blood flow, better preservation of the blood-brain barrier, and reduced brain swelling – all of which are critical in minimising stroke damage and aiding recovery.
Current Evidence and Limitations
While animal studies provide a strong foundation, the absence of completed human clinical trials testing cannabinoids for stroke recovery leaves a significant gap. Most of the evidence so far comes from rodent models, raising questions about how well these findings will translate to human patients.
The preclinical data itself reveals some complexities. For example, there are instances where blocking CB1 and CB2 receptors – rather than activating them – produced better outcomes. In certain models, mice lacking both receptors showed improved recovery after middle cerebral artery occlusion. This suggests that the effects of the endocannabinoid system (ECS) on stroke recovery may vary depending on the phase of the condition, with both receptor activation and inhibition showing benefits under different circumstances.
Timing also appears to be a crucial factor. In one study, CB2 agonists given as a pretreatment before stroke onset reduced neurodegeneration. However, when the same drugs were administered 2–5 days after ischaemia, their protective effects were no longer observed. Additionally, some cannabinoids induce hypothermia, which complicates the interpretation of whether their neuroprotective effects are due to ECS modulation or temperature changes.
Reviews published between 2022 and 2024 emphasise that, while cannabinoids show promise in animal studies, they remain experimental and should not replace established stroke treatments like thrombolysis, thrombectomy, or rehabilitation. The gap between preclinical and clinical applications is substantial, with variability in compounds, dosing, timing, and outcome measures across studies. Until robust randomised controlled trials are conducted in human stroke survivors, cannabinoids should be viewed as a potential avenue for future research rather than a current treatment option.
sbb-itb-24979b8
Medical Cannabis in Post-Stroke Care
Post-Stroke Symptoms That May Be Managed by Medical Cannabis
While medical cannabis is not approved for treating acute strokes, it shows potential in easing some lingering post-stroke symptoms. For instance, chronic post-stroke pain, which affects around 30–50% of patients, might be reduced by cannabinoids. These compounds interact with CB1 and CB2 receptors, helping to lower neuroinflammation and ease discomfort. Spasticity, experienced by about 20–40% of stroke survivors, could also benefit from the muscle-relaxing properties of cannabinoids.
Sleep issues are another common challenge after a stroke. Early research suggests that cannabinoids might improve sleep quality by alleviating stress and influencing astrocyte metabolism. Additionally, mood disorders like depression and anxiety, which often follow a stroke, could be addressed through the endocannabinoid system’s neuroprotective role. However, it’s essential to note that most of this evidence comes from preclinical studies, and there are no randomised controlled trials directly confirming the effectiveness of cannabis for post-stroke symptoms. Even so, these findings hint at a potential complementary role for medical cannabis alongside traditional rehabilitation methods.
Legal and Clinical Pathways in the UK
In the UK, medical cannabis has been available as a prescription-only treatment since November 2018, classified under Cannabis-Based Products for Medicinal Use in Humans (CBPMs). Only specialist doctors registered with the General Medical Council (GMC) can prescribe these treatments, typically when other therapies have failed to provide sufficient relief. UK regulations currently limit NHS prescriptions to specific conditions like treatment-resistant epilepsy, chemotherapy-induced nausea, and spasticity in multiple sclerosis. This means that for post-stroke symptoms such as pain or mood disturbances, patients generally need to seek treatment through private clinics.
For example, Nabiximols – a THC/CBD oromucosal spray – has a UK licence for managing spasticity in multiple sclerosis but is not officially approved for post-stroke use, making its application in this area off-label. The clinical process often begins with an eligibility assessment to confirm the diagnosis and review past treatments. This is followed by consultations with GMC-approved specialists, who may prescribe customised formulations, often starting with low-dose THC:CBD combinations. These prescriptions are filled through specialist pharmacies, with home delivery options available. Regular follow-ups help monitor side effects and adjust dosages as needed, especially given the cardiovascular considerations crucial for stroke survivors.
Elios Clinics: Patient-Focused Medical Cannabis Care
Elios Clinics specialises in offering medical cannabis treatments for neurological conditions, including post-stroke symptom management. The clinic provides free eligibility assessments via video consultations with GMC-approved doctors, removing the need for a GP referral. This approach prioritises patient convenience while leveraging the potential benefits of cannabinoids in supporting neuroprotection.
Treatment plans at Elios Clinics are tailored to individual needs. For instance, a CBD-dominant formulation might be suggested for anxiety or mood disorders, while a balanced THC/CBD preparation could be used to address pain and spasticity. These formulations are carefully titrated, starting with low doses to ensure safety and effectiveness. Medications are delivered directly to patients’ homes or local pharmacies, and regular follow-ups – typically every four to six weeks – allow for adjustments based on how well the treatment is working, any side effects, and safety checks like blood pressure monitoring. Elios Clinics also collaborates with patients’ healthcare teams, including GPs and rehabilitation specialists, to ensure comprehensive care.
Patients can choose from subscription plans (£60 quarterly or £20 monthly for a year, including an initial consultation and four follow-up sessions) or a pay-as-you-go option (£50 per consultation). This structured approach aims to deliver effective symptom relief while addressing the safety needs of stroke survivors.
Risks, Limitations, and Practical Considerations for Stroke Survivors
This section delves into the safety and practical aspects of using medical cannabis in stroke recovery, focusing on potential risks and strategies to minimise them.
Safety Considerations for Stroke Survivors
Stroke survivors face unique challenges when considering medical cannabis, particularly due to cardiovascular and cognitive vulnerabilities. Activation of CB1 receptors by THC can lead to effects like tachycardia, hypotension, and vasodilation, which might worsen post-stroke ischaemia. Additionally, CB1 activation has been linked to increased inflammation and altered blood flow, while studies in rodents have shown that blocking both CB1 and CB2 receptors can reduce inflammation and infarct size.
Cognitive concerns are another critical factor. THC-dominant products may impair memory and executive function by inhibiting voltage-sensitive calcium channels, potentially aggravating existing cognitive deficits caused by the stroke. Drug interactions also pose risks. For example, CBD inhibits CYP450 enzymes, which could elevate levels of anticoagulant and antiplatelet medications, increasing the likelihood of bleeding. Similarly, THC may amplify the sedative effects of antihypertensives or antidepressants, making careful dosing essential for patients juggling multiple medications.
These potential risks underscore the importance of cautious, well-monitored use, as outlined in the next sections.
Practical Tips for Medical Cannabis Use
To manage these risks effectively, certain dosing and administration strategies can be employed:
- CBD-dominant formulations are often safer for stroke survivors due to their minimal psychoactive effects. Research suggests cannabinoids may reduce infarct size, but THC should be introduced cautiously, starting with low doses (2.5–5 mg) to minimise cardiovascular stress.
- Balanced THC:CBD ratios may help with mood-related symptoms, though cognitive effects should be closely monitored.
- Sublingual oils or patches are preferred for their controlled absorption. If THC is necessary, begin with low doses to address severe symptoms. Avoid inhalation methods, as they can trigger tachycardia, and be cautious with edibles due to their delayed onset, which increases the risk of accidental overdosing, especially in those with cognitive impairments.
- Regularly track symptoms using standardised scales like the Visual Analogue Scale, Modified Ashworth Scale, or PHQ-9 to assess changes in pain, spasticity, and mood.
Monitoring and Adjusting Treatment Plans
Personalised treatment adjustments are vital to achieving the neuroprotective benefits discussed earlier. Regular reviews every 2–4 weeks are recommended to identify side effects such as hypotension or cognitive decline. Key metrics like blood pressure, heart rate, and cognitive function (using tools like the MoCA score) should be monitored consistently. Dosages should be increased gradually – by 10–20% every two weeks – if symptoms persist, and formulations should be modified if adverse effects occur.
"Our specialist clinicians at Elios Clinics are working hand in hand with your GP or other health professionals, and are always available in case you have any questions."
Collaboration between GMC-approved doctors and a patient’s existing healthcare team is essential for comprehensive monitoring. Patients are encouraged to document changes in pain, mood, and physical abilities to provide clear feedback during follow-up consultations. If adverse effects like confusion, hypotension, or falls occur, treatment plans should be revisited or discontinued.
It’s important to highlight that no human clinical trials have yet evaluated the use of cannabinoids in stroke recovery. Current evidence comes from preclinical models, and findings remain inconsistent.
Conclusion
This article delved into the intricate role of the endocannabinoid system in stroke recovery. Research suggests that this system plays a significant part in influencing stroke outcomes. Preclinical evidence indicates that modulating endocannabinoid receptors can reduce infarct size and improve functional recovery. For instance, a 2015 meta-analysis of 34 preclinical studies revealed that cannabinoids reduced infarct volume and enhanced functional outcomes in experimental stroke models. Cannabinoids like CBD, THC, and MAGL inhibitors have shown promise in rodent studies, helping to limit excitotoxicity, reduce neuroinflammation, and promote neuronal recovery.
However, human clinical trials evaluating cannabinoids for stroke recovery are non-existent. Current findings are primarily based on preclinical studies, which often yield conflicting results influenced by factors such as timing, compound type, and dosage. This underscores the complexity of the endocannabinoid system and its potential therapeutic applications.
For stroke survivors considering medical cannabis, personalised care is crucial. Treatment plans should take into account factors like the location of the infarct, timing of intervention, existing health conditions, and other medications. GMC-approved doctors can design tailored approaches to manage symptoms such as spasticity, chronic pain, or mood disturbances while minimising risks like cardiovascular strain or drug interactions.
"Our specialist doctors are among the highly trained doctors in the sphere of medical cannabis. They are ready to help you understand the potential benefits and possible risks… provide a specifically tailored treatment plan and support you all through the way." – Elios Clinics
While preclinical findings suggest notable neuroprotective benefits, translating these insights into clinical practice demands rigorous human trials. Until such evidence emerges, the use of medical cannabis in post-stroke care should be approached cautiously. Decisions should be informed and made collaboratively between patients, specialist clinicians, and healthcare teams. Ongoing research and well-structured clinical trials are essential to transform these preclinical discoveries into effective, personalised treatments.
FAQs
What role does the endocannabinoid system play in stroke recovery?
The endocannabinoid system is central to stroke recovery, as it helps manage inflammation, encourages neuroprotection, and aids in neural repair. These functions work together to reduce brain damage and enhance the recovery process following a stroke.
By tapping into the body’s own healing capabilities, the endocannabinoid system has the potential to improve recovery outcomes, bringing optimism to those undergoing rehabilitation.
What are the possible benefits and risks of using cannabinoids during stroke recovery?
Cannabinoids might provide several benefits for stroke recovery, including pain relief, reducing inflammation, enhancing sleep quality, and addressing mental health challenges like depression and anxiety. These effects are tied to the interaction between cannabinoids and the body’s endocannabinoid system, which helps regulate many essential functions.
That said, there are potential risks to be aware of. These can include dizziness, cognitive difficulties, or possible interactions with other medications. Since research into the long-term effects of cannabinoids is still developing, it’s essential to seek guidance from a qualified healthcare professional to determine if this treatment is suitable and safe for your individual situation.
Why are clinical trials important for developing endocannabinoid-based stroke treatments?
Clinical trials play a crucial role in confirming that therapies based on the endocannabinoid system for stroke recovery are both safe and effective. These trials involve testing treatments on human participants to gather solid evidence about their impact. They help establish the right dosage, uncover possible side effects, and verify how well the therapies work.
Adhering to rigorous medical research standards, clinical trials ensure that any new treatment meets top safety and quality requirements before it becomes accessible to patients on a larger scale.

