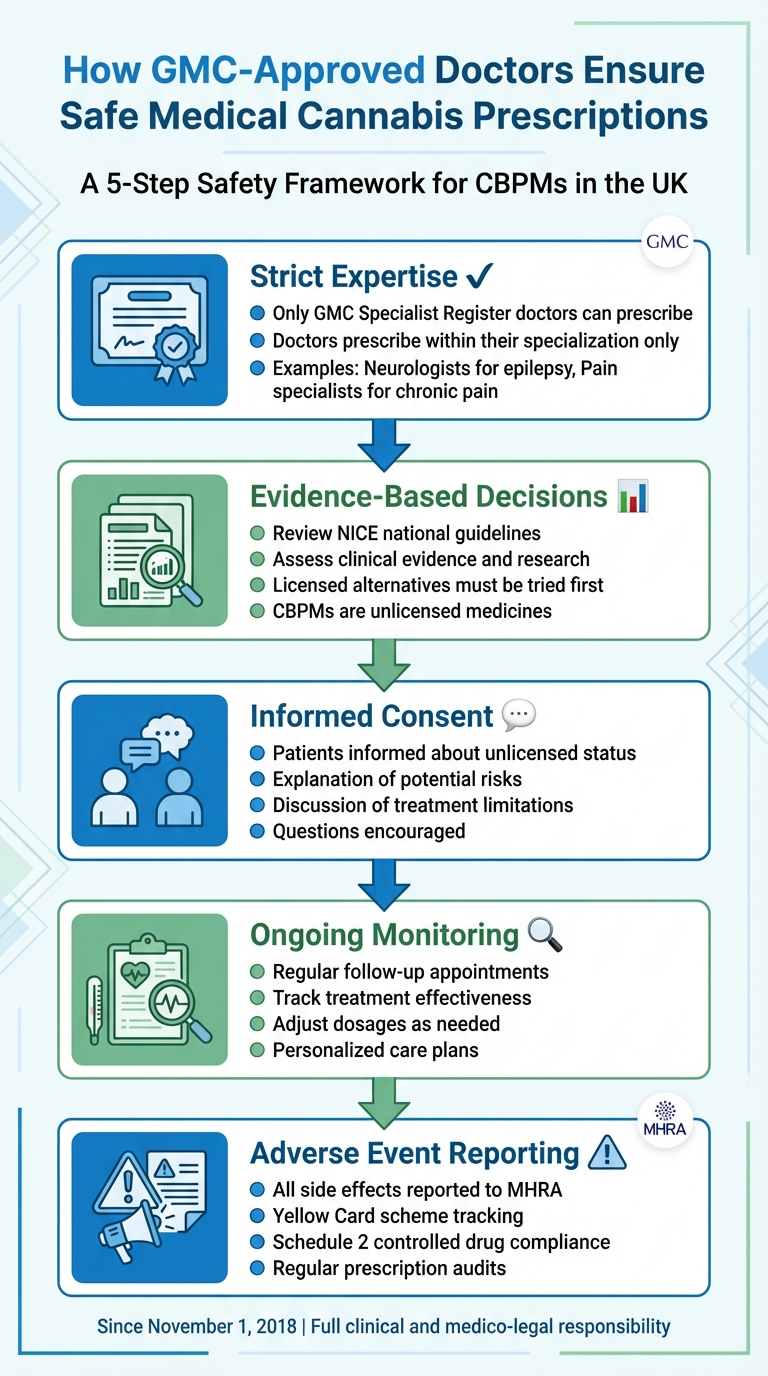
Medical cannabis prescriptions in the UK are tightly regulated to ensure patient safety. Only doctors listed on the General Medical Council‘s (GMC) Specialist Register can prescribe cannabis-based products for medicinal use (CBPMs), which are mostly unlicensed medicines. These specialists take full responsibility for assessing risks, tailoring treatment plans, and monitoring patients throughout their care. Here’s how they ensure safety:
- Strict Expertise: Doctors prescribe only within their area of specialisation (e.g., neurologists for epilepsy, pain specialists for chronic pain).
- Evidence-Based Decisions: They rely on national guidelines, such as those by NICE, and thorough clinical evidence to justify prescriptions.
- Informed Consent: Patients are fully informed about the unlicensed nature of CBPMs, potential risks, and treatment limitations.
- Ongoing Monitoring: Follow-up care ensures treatment effectiveness, with adjustments made as needed.
- Adverse Event Reporting: Any side effects are reported to the MHRA through the Yellow Card scheme to track safety concerns.
This rigorous process ensures that CBPMs are prescribed responsibly, prioritising patient wellbeing while adhering to UK regulations.
How GMC-Approved Doctors Ensure Safe Medical Cannabis Prescriptions in the UK
The Role of GMC-Approved Doctors in Cannabis Prescriptions
What Does GMC Approval Mean?
Being listed on the General Medical Council’s (GMC) Specialist Register is no small feat. It signifies years of rigorous training and a proven level of expertise. The GMC maintains this register to ensure that doctors who manage complex or high-risk treatments have the necessary qualifications and experience. When it comes to prescribing medical cannabis, this level of expertise is crucial.
"The legislation in the UK has restricted the decision to prescribe CBPMs to doctors on our Specialist Register." – General Medical Council
This requirement is particularly critical because most cannabis-based products for medicinal use (CBPMs) in the UK are unlicensed. They haven’t undergone the stringent safety and quality assessments required for licensed medicines, which means there are uncertainties about their long-term safety and effectiveness. Specialists are uniquely equipped to weigh these risks and benefits. For instance, a neurologist treating epilepsy approaches treatment decisions differently than a pain specialist managing chronic pain. This tailored expertise ensures that clinical decisions are made with precision and care.
Why Specialist Oversight Matters for Patient Safety
Specialist oversight isn’t just a formality – it’s a safeguard. These doctors have the training to deeply understand the conditions they treat and to determine when conventional treatments have been thoroughly explored. They are also adept at spotting potential interactions, contraindications, or warning signs that might escape the notice of less experienced clinicians.
"Treating patients with unlicensed medicines poses a higher risk than with a licensed medicine as they may not have been assessed for safety, quality, and efficacy." – Care Quality Commission
From the moment a specialist prescribes medical cannabis, they assume full clinical and medico-legal responsibility. This includes monitoring the patient’s progress, adjusting treatment plans if side effects arise, and reporting any adverse effects to the Medicines and Healthcare products Regulatory Agency (MHRA) via the Yellow Card scheme. This comprehensive approach ensures patients receive ongoing, expert care throughout their treatment.
At Elios Clinics, every medical cannabis prescription is handled by GMC-approved doctors who operate strictly within their areas of expertise. This ensures that treatment decisions are grounded in evidence and tailored to meet each patient’s unique needs.
Clinical Decision-Making and Evidence-Based Prescriptions
Assessing Clinical Evidence for Medical Cannabis
Doctors approved by the General Medical Council (GMC) prescribe medical cannabis only after thoroughly reviewing its safety and effectiveness. This process involves evaluating the latest research, national guidelines, and findings from international studies to ensure informed decision-making.
In the UK, the National Institute for Health and Care Excellence (NICE) provides a structured framework for prescribing cannabis-based medicines. NICE guidelines specify the conditions where these treatments might be suitable, such as spasticity caused by multiple sclerosis (MS), chemotherapy-induced nausea, and severe treatment-resistant epilepsy. For instance, NICE recommends using a THC:CBD spray for MS-related spasticity when other treatments fail. Similarly, cannabidiol (CBD) combined with clobazam is advised for severe epilepsy syndromes like Lennox-Gastaut or Dravet. However, NICE discourages prescribing cannabis-based medicines for managing chronic pain in adults unless within the context of clinical trials.
"Doctors must be satisfied there is sufficient evidence or experience to support an unlicensed medicine’s safety and efficacy." – General Medical Council
Unlicensed cannabis-based treatments are not considered first-line options. Licensed alternatives must always be explored first, and any clinical decision must be carefully documented in alignment with evidence-based practices.
Once this thorough evidence review is complete, doctors develop treatment plans tailored to each patient’s unique needs and circumstances.
Tailored Treatment Plans Based on Patient Needs
GMC-approved doctors craft prescriptions based on a detailed assessment of the patient’s condition. This involves evaluating the severity of symptoms, how they affect daily life, and whether conventional treatments have been effective. The evidence gathered during the clinical review directly informs these personalised care plans.
"When exercising their judgement, professionals and practitioners are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients." – NICE
Each treatment plan is designed with the patient’s medical history and current condition in mind. At Elios Clinics, this patient-focused approach ensures that every prescription is firmly rooted in clinical evidence. This methodology allows for ongoing monitoring and reinforces safety measures throughout the treatment process.
Informed Consent and Patient Education
Providing Clear Information to Patients
Doctors approved by the General Medical Council (GMC) make it a priority to inform patients that most medical cannabis products are unlicensed, meaning they haven’t undergone standard safety assessments. They explain the psychoactive nature of THC compared to the non-psychoactive CBD, discuss potential side effects, highlight the limitations of long-term evidence, and encourage patients to ask questions or even seek a second opinion.
"The exchange of information between medical professionals and patient is essential to good decision making. Serious harm can result if patients are not listened to, or if they are not given the information they need." – General Medical Council
This collaborative approach ensures that patients’ individual needs, preferences, and concerns are taken into account. After these discussions, doctors carefully document all aspects of the patient’s consent to ensure clarity and accountability.
Documenting Consent and Clinical Decisions
Once informed discussions have taken place, recording patient consent becomes a legal necessity. Doctors must document every aspect of the treatment conversation, including the justification for prescribing an unlicensed cannabis-based medicinal product (CBPM). This not only ensures transparency but also supports evidence-based decision-making.
The documentation should include clear explanations of why the medicine is unlicensed, how it addresses the patient’s unmet clinical needs, and any deviations from national guidelines. Such detailed record-keeping plays a vital role in minimising incidents involving controlled drugs and enhancing patient safety. At Elios Clinics, maintaining comprehensive records ensures that every prescription is transparent, well-justified, and adheres to GMC standards.
sbb-itb-24979b8
Follow-Up Care and Safety Monitoring
Regular Monitoring and Dose Adjustments
A GMC-approved doctor is responsible for overseeing follow-up care to track how well the treatment is working and to monitor for any side effects. This step is especially important for cannabis-based medicinal products (CBPMs), as these are mostly unlicensed and lack the extensive long-term safety data that conventional medicines have.
After the initial assessment, treatment plans often need fine-tuning. Adjustments are made collaboratively with the patient, taking into account their unique needs and lifestyle. Prescriptions are reviewed regularly to ensure the dosage aligns with the patient’s current health status. Any changes to the treatment plan must be grounded in solid evidence or clinical experience, and expert advice is sought when necessary.
"The prescribing doctor has responsibility for… overseeing a patient’s care and any follow‑up treatment [and] continuing to monitor the effectiveness and side‑effects of the prescribed medicine." – Care Quality Commission
At Elios Clinics, follow-up appointments are a key part of every treatment plan. These sessions allow doctors to monitor progress and make necessary dosage adjustments to enhance treatment effectiveness while prioritising safety.
Adverse Event Reporting and Compliance
In addition to regular monitoring, stringent adverse event reporting ensures treatment remains safe. GMC-approved doctors are legally required to report all adverse reactions to the Medicines and Healthcare products Regulatory Agency (MHRA) through the Yellow Card scheme. This national system helps identify potential risks associated with unlicensed medicines. Importantly, doctors must report any suspected side effects, even if they are not entirely certain of the cause.
"All adverse reactions should be reported to the Medicines and Healthcare products Regulatory Agency’s Yellow Card scheme." – General Medical Council
Since CBPMs are classified as Schedule 2 controlled drugs under the Misuse of Drugs Regulations 2001, they are subject to strict regulatory oversight. Healthcare providers must conduct regular audits of CBPM prescriptions to ensure both safety and compliance. All clinical decisions must be documented clearly, accurately, and legibly in medical records.
Conclusion
Prescribing medical cannabis in the UK goes far beyond simply writing a prescription. GMC-approved specialists bring together clinical expertise, legal responsibility, and stringent safety measures to ensure every treatment decision is carefully considered. From the first consultation to ongoing monitoring, these specialists make sure that cannabis-based medicinal products (CBMPs) are prescribed only after all licensed alternatives have been explored and when there is solid evidence to justify their use for a specific condition. This cautious and well-regulated approach reflects the principles we’ve discussed throughout.
Supporting these clinical practices is a strong regulatory framework designed to prioritise patient safety. Since 1 November 2018, prescribing doctors have been required to follow strict guidelines, including adhering to specialist training, keeping comprehensive records, and relying on evidence-based methods. This framework not only safeguards patients from the risks associated with unlicensed medicines but also ensures that treatments are carefully monitored and scientifically grounded.
"The prescriber to be responsible for the prescriptions they sign. It further requires that prescribers only prescribe drugs when they have adequate knowledge of the patient’s health, are competent to prescribe for that clinical condition and must be satisfied that the drugs serve the patient’s need." – General Medical Council
Another critical aspect of safe cannabis prescribing is informed consent and consistent monitoring. Patients are made fully aware of the unlicensed status of CBPMs and the limited long-term evidence, allowing them to make educated decisions about their treatment. Regular follow-ups, combined with adverse event reporting through the Yellow Card scheme, help address any concerns promptly and maintain patient safety.
FAQs
Why can only GMC-approved doctors prescribe medical cannabis?
In the UK, only doctors registered on the GMC Specialist Register are permitted to prescribe medical cannabis. This regulation ensures that such prescriptions are managed by professionals with the necessary specialised training and expertise to handle these high-risk, mostly unlicensed medications responsibly.
Doctors approved by the GMC adhere to stringent guidelines designed to reduce the risks of misuse, harm, or diversion. Their priority is patient safety, ensuring that cannabis-based treatments are used responsibly. By tailoring prescriptions to each individual’s needs, they strive to provide care that is both safe and effective.
How do GMC-approved doctors ensure the safe use of medical cannabis?
Doctors approved by the GMC follow strict safety protocols when prescribing medical cannabis. They only prescribe these unlicensed cannabis-based medicines within their specific area of expertise and after confirming there is enough evidence or clinical experience to support the product’s safety and effectiveness.
These doctors take full accountability for their prescriptions, ensuring patients are fully informed that the medicine is unlicensed. They also provide continuous care, closely monitoring how patients respond to the treatment and recording any side effects to ensure the ongoing safety and suitability of the medication.
How are patients monitored while using medical cannabis?
Patients prescribed medical cannabis receive careful monitoring to prioritise their safety and ensure the treatment is working as intended. This involves regular follow-up appointments, which can take place in-person, over the phone, or through video consultations. During these sessions, doctors review symptoms, check for any side effects, and make adjustments to dosages based on the patient’s progress and feedback.
In addition to monitoring, doctors offer educational materials to help patients gain a better understanding of their treatment. If necessary, they may also connect patients with other healthcare specialists for further support. This thorough and personalised approach ensures the treatment remains both safe and effective throughout the patient’s journey.
