Medical cannabis is legal in the UK, but confusion and myths still dominate public perception. Despite regulations and growing research, many people self-medicate illegally, unaware of safer, prescribed options. Misunderstandings stem from stigma, media focus on recreational use, and poor public education.
Key points include:
- Research exists: Over 30 trials and NHS data confirm benefits for chronic pain, epilepsy, and more.
- Safety: Addiction risk is low under medical supervision, and it’s far safer than opioids.
- Beyond pain relief: Treats anxiety, PTSD, multiple sclerosis, and seizures.
- Access: Only specialists can prescribe, with clinics offering consultations and personalised plans.
Patients and doctors must rely on evidence, not myths, to explore cannabis-based treatments effectively.
The Science and Health Effects of Medical Cannabis
Common Myths About Medical Cannabis
Medical Cannabis vs Traditional Medications: Safety and Addiction Risk Comparison
Even with legalisation, misconceptions about medical cannabis persist. These misunderstandings often prevent patients and healthcare providers from fully exploring its potential as a treatment option. Let’s tackle three common myths and compare them with the latest evidence.
Myth: There’s No Scientific Research on Medical Cannabis
Contrary to popular belief, medical cannabis is supported by extensive research. For instance, NICE guidelines are informed by evidence from 20 randomised controlled trials (RCTs) investigating its use for chronic pain. A comprehensive 2021 systematic review in the BMJ analysed 32 RCTs involving over 5,000 adult participants. Beyond chronic pain, studies have explored its role in treating conditions like intractable nausea, spasticity, and severe treatment-resistant epilepsy.
In April 2022, the NHS introduced the Medicinal Cannabis Patient Registry to collect real-world data on prescribing patterns and patient outcomes. Additionally, products such as Sativex, Epidyolex, and Nabilone have met rigorous safety and efficacy standards in the UK. To encourage even more research, the NIHR has issued a "Themed Call" for proposals on medical cannabis studies. Clearly, the claim that no scientific evidence exists is unfounded.
Myth: Medical Cannabis Is Dangerous or Causes Addiction
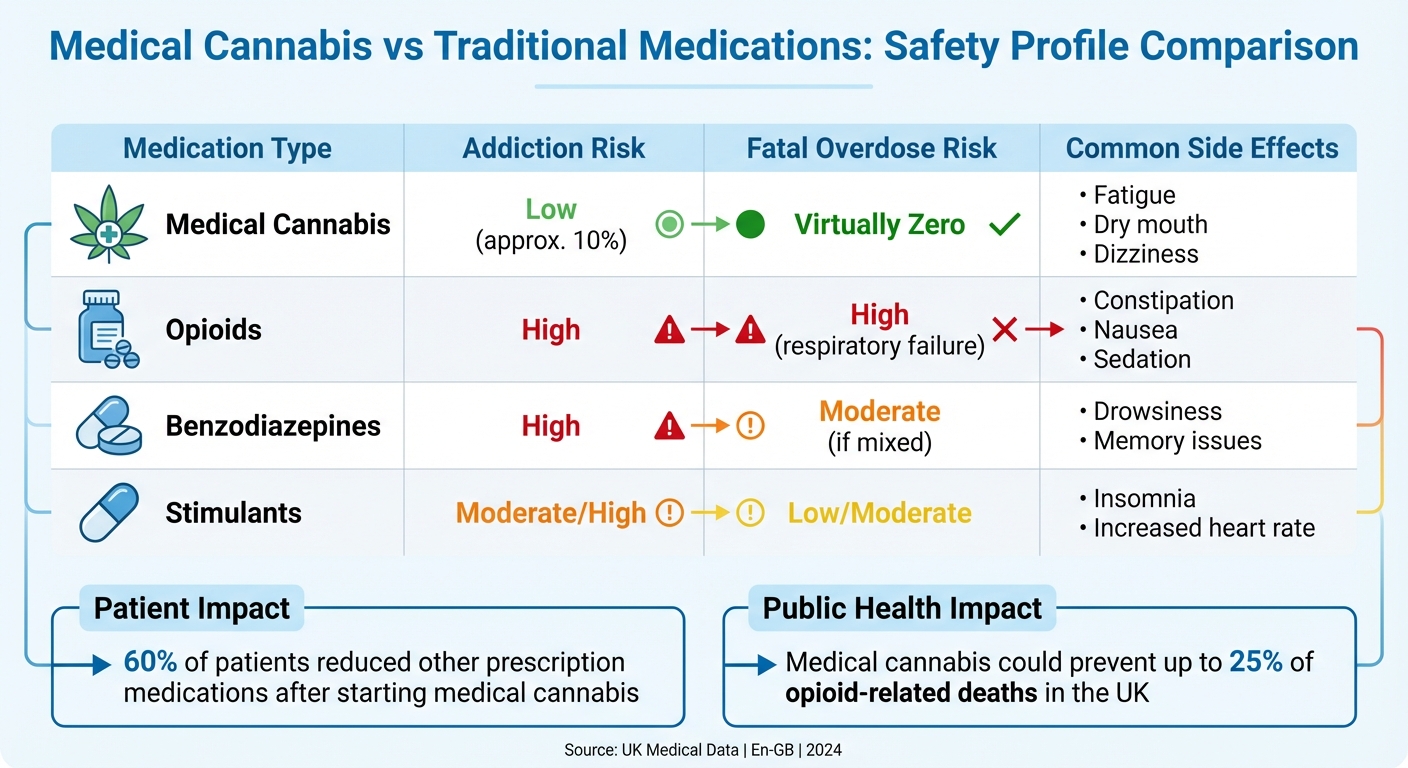
Another misconception is that medical cannabis poses significant risks, including addiction. However, the likelihood of addiction is extremely low, especially for patients without a history of substance misuse. About 10% of users may experience withdrawal symptoms if they stop abruptly, a figure much lower than for many commonly prescribed medications. When used under clinical supervision, the risk is even smaller, and medical cannabis is far less addictive than alternatives like opioids or benzodiazepines.
Unlike many traditional drugs, fatal overdoses from cannabis are virtually impossible. Research estimates that a person would need to consume an implausible 6,000 kg within 15 minutes to reach a lethal dose. In contrast, opioids carry a high risk of fatal respiratory depression, and benzodiazepines are notorious for dependency issues.
| Medication Type | Addiction Risk | Fatal Overdose Risk | Common Side Effects |
|---|---|---|---|
| Medical Cannabis | Low (approx. 10%) | Virtually Zero | Fatigue, dry mouth, dizziness |
| Opioids | High | High (respiratory failure) | Constipation, nausea, sedation |
| Benzodiazepines | High | Moderate (if mixed) | Drowsiness, memory issues |
| Stimulants | Moderate/High | Low/Moderate | Insomnia, increased heart rate |
Medical cannabis may even reduce the harm caused by other drugs. Evidence suggests that replacing opioids with cannabis could prevent up to 25% of opioid-related deaths in the UK each year. A 2023 survey revealed that 60% of patients reduced their use of other prescription medications after starting a medical cannabis treatment plan. This demonstrates that medical cannabis offers a safer alternative to many conventional treatments.
Myth: Medical Cannabis Only Treats Pain
While pain management is a well-known application, medical cannabis has a much broader therapeutic range. It is also used to address psychiatric conditions such as anxiety, PTSD, ADHD, and depression, as well as neurological disorders like epilepsy, multiple sclerosis, and autism. Additionally, it has shown promise for gastrointestinal issues, including inflammatory bowel disease.
For example, Epidyolex is licensed for severe seizure disorders like Lennox-Gastaut and Dravet syndromes, and Sativex is approved for spasticity in multiple sclerosis. Beyond these conditions, its benefits extend to improving sleep and mental well-being. A 2025 PLOS Mental Health study of 124 patients with primary insomnia reported significant improvements in sleep quality (Single-Item Sleep Quality Scale scores rose from 2.66 to 5.67, p < 0.001), with only 8.87% experiencing mild side effects.
Similarly, a 2025 case series published in Brain and Behaviour followed 64 patients with Complex Regional Pain Syndrome (CRPS) for six months. Participants not only experienced pain relief but also reported better sleep and reduced anxiety (p < 0.050). In fact, 85% of patients in the Project Twenty21 registry are treated for chronic pain or anxiety disorders, highlighting the diverse therapeutic applications of medical cannabis. These findings confirm that its benefits go far beyond pain relief, offering a wide range of treatment possibilities.
What the Science Says About Medical Cannabis
Patient Outcomes from Clinical Data
The UK Medical Cannabis Registry (UKMCR) offers valuable insights into how medical cannabis impacts patients. A 2022 case series involving 190 chronic pain patients highlighted notable improvements in several areas at one, three, and six months. Patients reported better pain management (measured by the Brief Pain Inventory), reduced anxiety levels (tracked using GAD-7), and improved sleep quality. While 39.47% experienced side effects, the majority were mild (19.47%) or moderate (12.11%), with nausea being the most common complaint at just 5.8%. Similar trends have been observed in epilepsy studies.
"An association was identified between patients with chronic pain prescribed CBMPs and improvements in pain-specific and general HRQoL outcomes." – Michael Harris et al., UCL Discovery
In 2025, an analysis of 134 patients with treatment-resistant epilepsy found that 29.85% achieved a meaningful improvement in their Quality of Life in Epilepsy-31 scores after six months. Only 3.73% reported mild side effects such as fatigue or headaches. Another UK-based initiative, Project Twenty21, showed that 95.51% of sessions involving inhaled cannabis flower led to reduced symptom severity in conditions like anxiety and stress.
Licensed Cannabis Medicines in the UK
Currently, the UK has approved three cannabis-based medicines:
- Sativex: This 1:1 THC:CBD spray is prescribed for moderate to severe spasticity in multiple sclerosis patients who haven’t responded to other treatments. Continued use after an initial four-week trial depends on achieving at least a 20% reduction in spasticity symptoms.
- Epidyolex: A pure CBD product, it is approved for managing seizures in patients aged two and older with Lennox-Gastaut syndrome, Dravet syndrome, or tuberous sclerosis complex. Treatment is reviewed every six months and discontinued if seizure frequency doesn’t drop by at least 30%.
- Nabilone: This synthetic cannabinoid is used to treat chemotherapy-induced nausea and vomiting when standard antiemetics fail.
Clinical Trials vs. Patient Registry Data
Real-world registry data offers a broader perspective compared to traditional randomised controlled trials (RCTs). While RCTs focus on tightly controlled conditions with carefully selected participants, they often run for only a few weeks and exclude patients with comorbidities. In contrast, registries like Project Twenty21 capture long-term, real-world usage. By March 2022, this registry had collected data on 1,176 chronic pain patients – over 27% of the total studied in all RCTs for medical cannabis in chronic pain.
Sir Michael Rawlins, former chair of the MHRA and NICE, questioned the dominance of RCTs in medical research:
"Randomised controlled trials have been placed on an undeserved pedestal… They should be replaced by a diversity of approaches that involve analysing the totality of the evidence base."
Registry data reflects how patients use medical cannabis in their daily lives. For example, only 31.7% of Project Twenty21 participants used a single cannabis product, while 23.4% relied on three or more prescribed products – scenarios rarely addressed in controlled trials. Some registry studies have tracked patients for up to 18 months, offering insights into the long-term safety and effectiveness of medical cannabis that short-term RCTs cannot provide. Together, these data sources strengthen the scientific foundation for medical cannabis and help dispel lingering misconceptions.
sbb-itb-24979b8
How to Access Medical Cannabis in the UK
UK Medical Cannabis Regulations
Understanding the legal framework for medical cannabis is crucial for those considering it as a treatment option. Since 1 November 2018, certain cannabis-based products have been legal for prescription in the UK. However, strict rules govern who can prescribe them. Only doctors listed on the General Medical Council (GMC) Specialist Register are authorised to prescribe cannabis-based products for medicinal use. This means your GP cannot issue prescriptions for medical cannabis.
"The legislation in the UK has restricted the decision to prescribe CBPMs to doctors on our Specialist Register." – General Medical Council
Medical cannabis is only considered when there is an unmet clinical need – essentially, when other licensed treatments have failed or are deemed unsuitable. Most cannabis-based medicinal products are classified as Schedule 2 controlled drugs, which means they must be prescribed on a named-patient basis. Additionally, NHS England advises that prescribing decisions involve a multidisciplinary team to prioritise patient safety.
If you’re travelling with medical cannabis, ensure it remains in its original packaging with the pharmacist’s dispensing label. Carry your prescription and, ideally, a letter from your doctor. Any side effects should be reported through the MHRA Yellow Card Scheme.
These regulations aim to balance patient access with safety, ensuring treatments are provided through the appropriate channels.
Services Available at Elios Clinics
For those navigating the process, clinics like Elios Clinics simplify access to medical cannabis. Their approach starts with a free online eligibility assessment, where your health concerns, symptoms, and treatment history are reviewed to determine if you qualify for medical cannabis.
If eligible, you’ll have a consultation with a GMC-registered specialist consultant. This consultation, which can be conducted either at their Oxford clinic or via a secure video platform, involves a thorough review of your medical history. Based on this, a personalised treatment plan is created to address your specific needs. The availability of video consultations ensures that patients across the UK can access specialist care without the hassle of travel.
Elios Clinics also offers flexible payment plans to suit different budgets. Options include a quarterly subscription (£60), a monthly subscription (£20), or a pay-as-you-go consultation (£50 per session). Subscription plans cover an initial consultation, four follow-up appointments each year, and monthly prescriptions, all managed through an online patient dashboard. The clinic also provides ongoing support, including monitoring and adjusting treatments as needed.
Conclusion: Moving Beyond Myths to Evidence
What Patients and Doctors Should Know
Regulated medical cannabis stands apart from unpredictable black-market products. For instance, the THC content in illicit cannabis rose from 6.9% to 10.6% between 2010 and 2019, making such products unreliable and potentially unsafe.
Clinical studies highlight its potential benefits for specific conditions. Data shows that CBD can reduce seizures by 50% in certain forms of epilepsy. Additionally, a meta-analysis of 32 trials involving 5,174 adults found a 10% increase in meaningful pain relief when using non-inhaled cannabis compared to a placebo. As summarised in the BMJ:
"Moderate to high certainty evidence shows that non‐inhaled medical cannabis or cannabinoids results in a small to very small improvement in pain relief, physical functioning, and sleep quality among patients with chronic pain."
Interestingly, a 2023 survey by the Medical Cannabis Alliance revealed that 60% of patients reduced their use of other prescribed medications after starting cannabis-based treatments.
To make informed choices, patients should seek advice from GMC-registered specialists, opt for pharmaceutical-grade products rather than unregulated CBD supplements, and report any side effects through the MHRA Yellow Card Scheme. Healthcare professionals, on the other hand, should consult frameworks like the NICE guidelines (NG144) for evidence-based recommendations on conditions such as chronic pain, severe epilepsy, spasticity, and intractable nausea.
FAQs
How can I access medical cannabis legally in the UK?
In the UK, medical cannabis is legally available but strictly regulated. To access it, you need a prescription from a GMC-registered specialist doctor. This usually involves either a referral through the NHS or an evaluation at a private clinic to determine if you’re eligible for treatment.
When prescribed, the medication is supplied by a licensed pharmacy. It’s worth noting that medical cannabis is only considered for certain conditions, particularly when other treatments have failed. The entire process is closely monitored to maintain safety and high-quality standards.
What conditions can medical cannabis help manage besides chronic pain?
Medical cannabis has proven to be helpful in managing various conditions beyond just chronic pain. For instance, it is frequently prescribed to treat severe epilepsy, particularly in children, and is effective in reducing nausea and vomiting caused by chemotherapy. Additionally, it can ease muscle stiffness and spasms in individuals with multiple sclerosis. Early research also indicates it may assist in alleviating symptoms associated with Alzheimer’s disease, Crohn’s disease, and certain cancer-related complications.
Though studies are still underway, medical cannabis provides an alternative for patients who haven’t found relief with standard treatments. It’s essential to consult a qualified medical professional to assess whether it’s the right choice for your condition.
Is medical cannabis a safer alternative to opioids for managing pain?
Medical cannabis is frequently seen as a safer alternative to opioids for managing chronic pain. It presents a reduced risk of addiction and tends to cause fewer severe side effects, which can make it a more suitable option for many patients over time.
Research, including scientific studies and systematic reviews, has underscored its promising safety profile for pain management. Unlike opioids, which come with serious risks like dependency and overdose, medical cannabis provides a more measured option when prescribed and monitored by healthcare professionals.