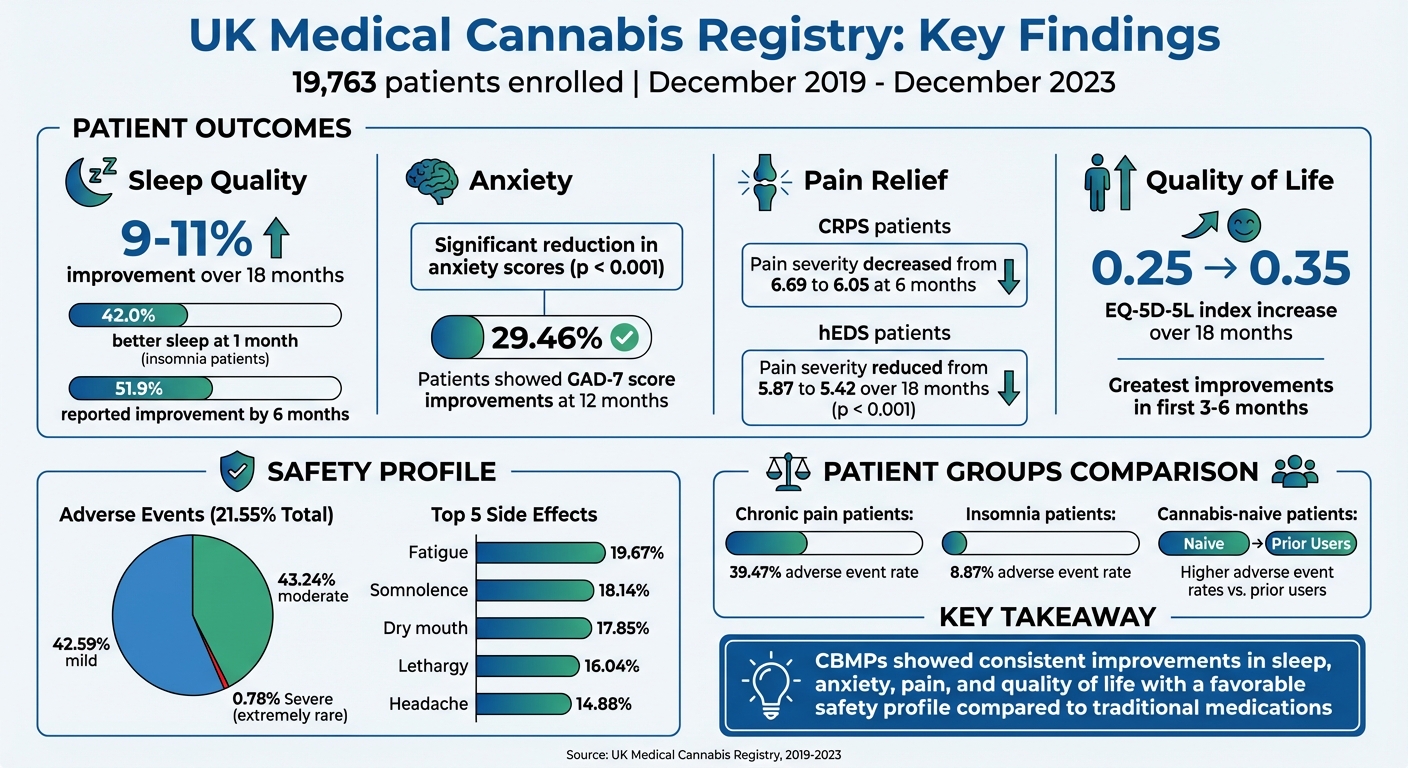
The UK Medical Cannabis Registry (UKMCR), launched in December 2019, is the largest anonymised database in the UK tracking outcomes of cannabis-based medicinal products (CBMPs). With 19,763 patients enrolled by December 2023, it provides real-world data on conditions like chronic pain and anxiety. Key findings include:
- Sleep and Anxiety: Sleep quality improved 9–11% over 18 months; anxiety scores dropped significantly (p < 0.001).
- Pain Relief: Conditions like CRPS and hEDS saw reductions in pain severity within months.
- Quality of Life: EQ-5D-5L scores showed gains, especially in the first 6 months.
- Safety: 21.55% experienced mild-to-moderate adverse events, with severe cases being rare.
CBMPs offer an alternative for patients unresponsive to other treatments, with data supporting better sleep, reduced pain, and improved mental health. However, more research is needed to address gaps like placebo comparisons and long-term safety.
UK Medical Cannabis Registry: Key Patient Outcomes and Safety Data
Long-Term Patient Outcomes: Main Results
Sleep Quality and Anxiety Improvements
Within the first month of treatment, patients experienced noticeable improvements in both sleep quality and anxiety, with these benefits remaining consistent over longer periods. Sleep quality saw an increase of 9%–11% between 1 and 18 months, while anxiety scores showed a significant reduction (p < 0.001). By the one-month mark, 42.0% of patients receiving treatment primarily for insomnia reported better sleep, increasing to 51.9% by six months. For anxiety, about 29.46% of patients showed improvements in their GAD-7 scores after 12 months. Interestingly, patients using inhaled dried flower reported greater improvements in both sleep and anxiety compared to those using other methods of administration.
These advancements in sleep and anxiety not only addressed immediate concerns but also contributed to broader gains in overall quality of life and mental health.
PTSD Symptoms and Quality of Life
Long-term treatment extended these early improvements, particularly in quality of life measures. A study involving 161 patients revealed that the EQ-5D-5L index value rose from 0.25 ± 0.28 at baseline to 0.35 ± 0.30 after 18 months of treatment. Similarly, the anxiety and depression subscale saw a reduction from 2.61 ± 1.23 to 2.39 ± 1.17 (p < 0.001).
Mary Dickinson from Imperial College London’s Medical Cannabis Research Group noted: "An association was identified between patients with HSD/hEDS with chronic pain and improvements in pain‐specific and general health‐related quality of life following the commencement of CBMPs".
The most dramatic improvements were observed during the first three to six months of therapy. After this period, results stabilised but remained significantly better than baseline even at 18 months.
Pain Reduction and Condition-Specific Outcomes
Pain severity showed marked reductions across various conditions, with noticeable changes as early as three months and sustained benefits over 18 months. For example, patients with complex regional pain syndrome (CRPS) reported a decrease in pain severity from 6.69 ± 1.42 at baseline to 6.05 ± 1.72 at six months (p < 0.050). Pain interference scores for these patients also improved, dropping from 7.21 to 6.18 during the same period. Similarly, individuals with hypermobile Ehlers-Danlos syndrome (hEDS) experienced a reduction in pain severity from 5.87 ± 1.42 to 5.42 ± 1.62 over 18 months (p < 0.001).
One notable case from 2025 highlighted a UK patient with hEDS who, within three months of starting treatment, successfully discontinued prescribed opioids – previously amounting to 220 mg of oral morphine equivalents per day. This was accompanied by increased mobility and reduced reliance on daily care.
Treatment commonly involved a mix of CBD-dominant oils and THC-dominant dried flower. For instance, median THC doses for CRPS patients increased from 12.50 mg/day at baseline to 117.86 mg/day at six months.
Lilia Evans from Imperial College London remarked: "This study represents the outcomes in individuals with complex regional pain syndrome prescribed cannabis‐based medicinal products. These suggest initiation of cannabis‐based medicinal products is associated with improvements in patient‐reported outcome measures".
Safety Profile and Adverse Events
Reported Adverse Events: Frequency and Severity
In addition to showing improvements in patient outcomes, the registry offers valuable insights into the long-term safety of cannabis-based medicinal products (CBMPs). A 12-month study involving 1,378 patients revealed that 21.55% experienced at least one adverse event, with 42.59% of these being mild and 43.24% classified as moderate. The most common side effects reported were fatigue (19.67%), somnolence (18.14%), dry mouth (17.85%), lethargy (16.04%), and headache (14.88%). These effects were generally short-lived and rarely led to patients discontinuing treatment. Severe adverse events were exceedingly uncommon – a separate analysis of 129 patients noted only one case (0.78%) of disabling insomnia, which lasted for four days.
Adverse event rates also differed across patient groups. For example, chronic pain patients reported a frequency of 39.47%, with nausea occurring in 5.8% of cases. In contrast, insomnia patients had a lower rate of 8.87%. Interestingly, individuals new to cannabis reported more adverse events compared to those who had prior exposure.
Dr Simon Erridge, Head of Research and Access at Sapphire Medical Clinics, commented: "Patients who were not consuming cannabis at baseline were more likely to experience adverse events".
Safety Comparison with Standard Medications
When compared to conventional treatments, the adverse events linked to CBMPs present a starkly different risk profile. Traditional medications for chronic conditions often carry significant risks. For instance, long-term use of NSAIDs is associated with dose-dependent risks like gastrointestinal ulcers and cardiovascular complications. Similarly, gabapentinoids have raised concerns due to potential misuse and dependency with extended use. Meanwhile, opioids are infamous for their risks of addiction, toxicity, and respiratory depression, and they lack strong evidence for effectively managing chronic pain.
In contrast, adverse events from CBMPs are typically mild to moderate in severity. As Mehmet Ergisi and colleagues noted, "This study demonstrated CBMP treatment to be associated with a relatively low incidence of severe adverse events in the medium-term".
More research, particularly involving active comparators, is needed to fully understand the long-term safety of CBMPs. However, the current data highlights their potential as a viable option for managing chronic conditions over extended periods.
Statistical Methods and Study Limitations
Regression Analysis and Outcome Predictors
To validate the reported patient outcomes, researchers utilised advanced statistical techniques. Paired t-tests were applied for parametric data, while Wilcoxon rank-sum tests were used for non-parametric data to compare patient-reported outcomes across 1, 3, 6, and 12 months against baseline values. The Shapiro-Wilk test assessed data normality, and all analyses were conducted using IBM SPSS Statistics version 27.
Univariate and multivariate logistic regression models were used to pinpoint predictors of clinically meaningful improvements. Factors such as age, gender, and the method of administration (oil versus dried flower) were analysed. Post hoc evaluations further explored outcomes at each follow-up interval. A p-value of < 0.050 consistently defined statistical significance. These analyses provide a foundation for understanding the long-term improvements observed, while also highlighting the need to address the limitations inherent in the study’s design.
Study Limitations and Research Gaps
Despite the detailed analysis, the study faces several limitations. Its observational nature restricts the ability to draw causal conclusions. The lack of a placebo or active comparator means it is difficult to definitively attribute observed changes to the use of CBMPs.
Another challenge lies in the potential influence of prior cannabis use. Many participants had previous exposure to cannabis, which could affect tolerance levels and the detection of adverse events. Additionally, the reliance on subjective patient-reported measures may introduce variability in outcome assessments.
The researchers emphasise the importance of conducting randomised controlled trials to establish causation more definitively. As noted in a clinical safety study, "Randomized controlled trials are still awaited to assess causation; however, real-world evidence can help inform current clinical practice, future trials, and is an important component of pharmacovigilance". Future research should incorporate active comparators, investigate long-term safety, and determine optimal dosing strategies to address these gaps effectively.
sbb-itb-24979b8
Dr Simon Erridge – UK Medical Cannabis Registry – July 2021
Conclusion
The UK Medical Cannabis Registry highlights the long-term benefits of cannabis-based medicinal products (CBMPs) for managing chronic conditions. Patients have reported improvements in areas like anxiety, sleep quality, pain management, and overall quality of life. What’s impressive is that many of these benefits became apparent within the first month of treatment and remained consistent over follow-up periods of up to 18 months. These findings help fill the gaps left by randomised controlled trials (RCTs) and provide valuable insights for clinical practice, supporting the potential inclusion of CBMPs within the NHS framework.
Safety data further supports the use of CBMPs, showing that adverse effects were mostly mild to moderate – especially when compared to traditional medications. For patients who haven’t found relief with at least two other licensed treatments, CBMPs offer a promising alternative. The registry also helps clinicians tailor treatments to individual patient needs, considering factors like patient profiles and administration methods. This patient-focused approach is evident in settings such as Elios Clinics, where real-world data is used to guide treatment strategies for chronic conditions across the UK.
FAQs
What does the UK Medical Cannabis Registry reveal about the long-term benefits of medical cannabis treatments?
The UK Medical Cannabis Registry has shed light on the long-term advantages of cannabis-based medicinal products for individuals dealing with chronic conditions. Among the reported benefits are lower anxiety levels, better sleep quality, and noticeable improvements in overall quality of life related to health.
Patients managing conditions such as ADHD and chronic pain have shared experiences of meaningful progress, including sustained relief and enhanced day-to-day functioning. These results emphasise the value of personalised, patient-centred treatment plans in achieving the best possible outcomes.
How safe are cannabis-based medicinal products compared to traditional medications?
Cannabis-based medicinal products (CBMPs) are often recognised for having a safety profile that compares well to many traditional medications. According to data from the UK Medical Cannabis Registry, CBMPs have shown potential in improving conditions like PTSD, anxiety, sleep disorders, and depression. When side effects do occur, they are generally mild and manageable.
Unlike many conventional treatments, which can come with a range of well-documented side effects, CBMPs provide an alternative that is seen as acceptable, particularly for patients whose conditions haven’t responded to standard therapies. That said, it’s crucial that these products are prescribed and closely monitored by qualified healthcare professionals to ensure they are both safe and effective.
What are the challenges of the UK Medical Cannabis Registry study?
The UK Medical Cannabis Registry study does face certain limitations, primarily due to its observational design. This type of study can’t establish definitive cause-and-effect links between medical cannabis treatments and patient outcomes. Additionally, relying on patient-reported data and routine clinical records introduces the possibility of biases or incomplete information, which could influence the accuracy of the results.
Another challenge lies in the variety of patients, conditions, and treatment approaches included in the registry. This diversity makes it hard to standardise findings or draw solid conclusions about the long-term safety and effectiveness of medical cannabis. Since the registry is still in its early stages, there’s also only limited data available on extended outcomes, underlining the importance of continued research to fully grasp the long-term effects of using medical cannabis.