GPs in the UK are increasingly encountering patients using or seeking cannabis-based medicinal products (CBPMs). Since the law changed on 1 November 2018, only specialist doctors on the GMC Specialist Register can prescribe CBPMs. These products are mostly accessed privately through over 40 clinics and 160 prescribers. GPs play a supporting role, managing patient histories, monitoring outcomes, and collaborating under shared care arrangements.
Key points:
- GPs cannot prescribe unlicensed CBPMs but may assist in monitoring under specialist-led care.
- CBPMs include licensed products like Sativex and Epidyolex, as well as unlicensed "specials."
- NHS prescriptions are rare, restricted to severe epilepsy, MS spasticity, and chemotherapy-related nausea.
- Most CBPMs are accessed privately for conditions like chronic pain, anxiety, and PTSD.
- GPs must ensure proper documentation, monitor treatment, and address risks like drug interactions and side effects.
This guide explains the GP’s role, legal framework, and how to work with specialist clinics like Elios Clinics to ensure safe and effective patient care.
Legal and Regulatory Framework
NHS vs Private Medical Cannabis Prescribing in the UK: Key Differences
How Medical Cannabis Is Classified in UK Law
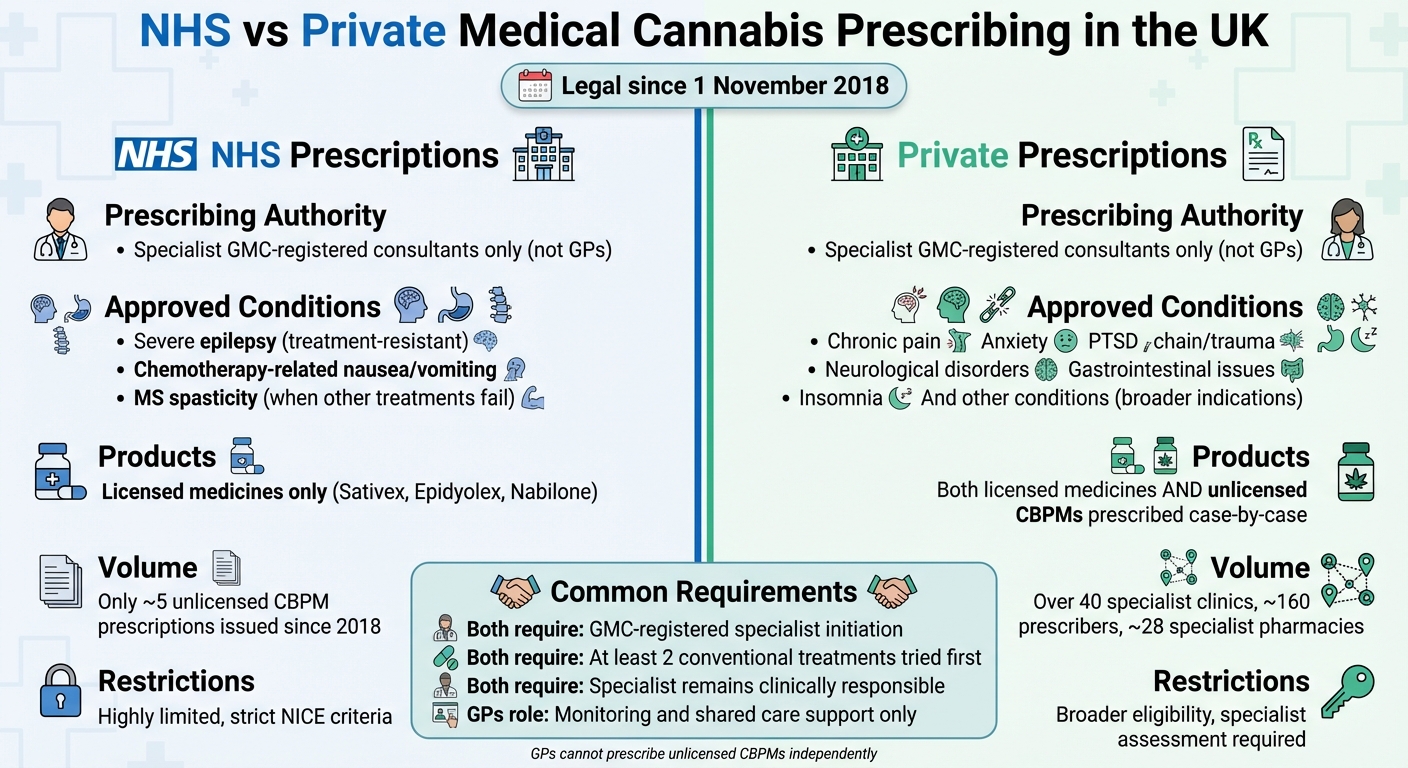
On 1 November 2018, cannabis-based products for medicinal use in humans (CBPMs) were rescheduled to Schedule 2 under the Misuse of Drugs Regulations 2001. This change allowed specialist doctors to prescribe these products, although cannabis itself remains a Class B controlled drug under the Misuse of Drugs Act 1971.
The legal framework distinguishes between licensed cannabis medicines – such as Sativex, Epidyolex, and Nabilone – which have been approved by the MHRA following clinical trials, and unlicensed CBPMs, which are prescribed as "specials". These unlicensed CBPMs meet MHRA quality standards but require additional documentation and safety protocols.
For GPs, it’s crucial to understand that over-the-counter CBD products marketed as wellness items are regulated by the Food Standards Agency as consumer goods or novel foods. These products cannot make medicinal claims and fall outside the CBPM framework entirely. This distinction is key to understanding prescribing roles within clinical practice.
Who Can Prescribe Medical Cannabis
Only specialist doctors listed on the GMC Specialist Register are authorised to prescribe CBPMs. These specialists typically work within multidisciplinary teams. GPs themselves cannot initiate unlicensed CBPMs but may play a role in monitoring patients under shared care arrangements.
NHS prescriptions for CBPMs are highly limited. According to NICE guidelines, NHS use is restricted to severe, treatment-resistant epilepsy, chemotherapy-induced nausea and vomiting, and spasticity in multiple sclerosis when other treatments have failed. Since the 2018 rescheduling, only around five unlicensed CBPM prescriptions have been issued within the NHS due to these strict criteria.
In contrast, most patients access CBPMs through private clinics. The UK now has over 40 specialist cannabis clinics and approximately 160 prescribers, with about 28 specialist pharmacies dispensing imported products. Private specialists assess patient eligibility, prescribe appropriate formulations, and coordinate supply through these dedicated pharmacies. Clinics such as Elios Clinics offer services like eligibility assessments, video consultations, and personalised treatment plans for conditions such as chronic pain, psychiatric disorders, and neurological issues.
| Prescribing Authority | NHS Indications | Private Prescribing |
|---|---|---|
| Specialist GMC-registered consultants only (not GPs) | Severe epilepsy, chemotherapy-related nausea/vomiting, MS spasticity (when other treatments fail) | Broader indications, including chronic pain, anxiety, PTSD, etc. |
| Licensed medicines (e.g., Sativex, Epidyolex, Nabilone) | As approved by MHRA/NICE | Unlicensed CBPMs prescribed case-by-case by specialists |
GPs must navigate these legal distinctions carefully, ensuring that shared care arrangements align with specialist treatment plans and that clinical records accurately reflect these details. This framework highlights the responsibilities of specialists and the structure of patient care, which is further supported by key guidance documents.
Key Guidance Resources for GPs
Several national guidelines provide essential information for GPs managing patients prescribed CBPMs. NHS England guidance outlines the legal definitions, prescribing roles, and commissioning expectations for cannabis-based medicinal products. The GMC prescribing standards stress the importance of evidence-based practice, proper handling of unlicensed medicines, and maintaining appropriate expertise.
NICE guidelines play a central role in shaping local formularies and limit NHS prescriptions to the specific conditions mentioned earlier. Meanwhile, the MHRA oversees the quality, safety, and monitoring of both licensed and unlicensed CBPMs. Additionally, the Medical Cannabis Clinicians Society (MCCS) Good Practice Guide offers profession-led advice on safe prescribing, risk evaluation, and monitoring, helping GPs interpret specialist recommendations.
When patients receive treatment through private clinics like Elios Clinics, effective communication from specialists becomes essential. Specialists must provide clear details about the treatment rationale, monitoring plans, and potential drug interactions. This ensures GPs can deliver safe, coordinated care while operating within their professional boundaries.
Clinical Responsibilities and Working with Clinics
GPs play a critical role in managing clinical and documentation tasks when working alongside specialists, adhering to the relevant legal framework.
What GPs Are Responsible For
GPs provide essential support in specialist-led CBMP care by identifying suitable patients, maintaining accurate records, and ensuring ongoing monitoring. However, the specialist remains ultimately responsible for clinical decisions.
The process starts with identifying patients whose symptoms persist despite optimised conventional treatments. This includes conditions like chronic neuropathic pain, multiple sclerosis spasticity, treatment-resistant epilepsy, PTSD, or anxiety. GPs need to confirm that prior therapies were used at appropriate doses and durations, check for any contraindications (e.g., pregnancy, significant cardiovascular issues, or a history of psychosis), and set realistic expectations before referring the patient to a specialist.
Once a specialist prescribes a CBMP, GPs must record the product in the GP prescribing system to enable safe co-prescribing and interaction checks. Their ongoing responsibilities include monitoring the patient’s general health – both physical and mental – managing other medications, and promptly alerting the specialist to any safety concerns.
When making referrals, GPs should include comprehensive and up-to-date information, such as clinical and medication histories (including controlled drugs and any recreational cannabis use), relevant test results, treatment history with reasons for discontinuation, and details on mental health, substance use, safeguarding concerns, or DVLA-related issues. This ensures the specialist can seamlessly integrate their treatment plan into the broader scope of patient care.
Shared Care Agreements Explained
Shared care agreements formalise the collaboration between specialists and GPs, outlining responsibilities for prescribing and monitoring unlicensed medicines like CBMPs. In these agreements, the specialist retains overall clinical responsibility but delegates specific tasks to the GP under clear protocols.
Typically, the specialist diagnoses the condition, initiates treatment, obtains the patient’s informed consent, and sets out the dosing, monitoring, and review schedule. The GP’s role involves prescribing repeat medications as per the specialist’s plan, monitoring adherence and side effects, conducting agreed checks (e.g., blood pressure, mental health reviews, pregnancy status), and reporting any concerns or adverse events back to the specialist.
Before entering into shared care, GPs need to ensure there’s a written protocol detailing the condition, product, dosage, monitoring requirements, and review criteria. They should also verify that the CBMP complies with national and local ICB policies, assess their ability to manage potential risks (e.g., sedation, cognitive changes, mood disturbances, or misuse), and confirm practical aspects like prescription duration and specialist review timelines. If anything is unclear, GPs can decline shared care and request clarification from the specialist or clinic.
NHS England guidelines specify that non-specialists can only prescribe CBMPs following specialist initiation and stabilisation, with the patient remaining under specialist care. Without a formal shared care agreement, GPs are not obligated to continue private prescriptions if they feel it is unsafe or beyond their expertise. In such cases, GPs should review national and local policies, inform the patient that prescribing remains the specialist’s responsibility, ensure proper coding of the CBMP in the GP record, and continue routine care and safety monitoring while maintaining communication with the prescribing clinic.
Collaborating with Elios Clinics
In line with these shared care principles, regulated clinics like Elios Clinics offer structured assessments through GMC-registered specialists. These assessments, often conducted via video consultations, focus on conditions such as chronic pain, psychiatric disorders, and neurological issues where standard treatments have not succeeded. Specialists at Elios Clinics provide GPs with detailed letters outlining the condition, product type (e.g., oil or flower), THC:CBD ratio, dosing plan, monitoring recommendations, treatment rationale, and evidence of informed consent.
Elios Clinics’ specialists work "hand in hand" with GPs and other healthcare professionals to ensure seamless patient care, and they are "always available" to address questions about treatment plans, potential benefits, or risks associated with medical cannabis.
This open communication helps GPs integrate the specialist’s treatment plan into the patient’s broader medical history and overall care. While patients can book an initial appointment with Elios Clinics without a GP referral, providing complete medical records at the referral stage enables a safer and more informed specialist assessment.
Successful collaboration relies on timely clinic updates after each specialist review, including changes to dosage, formulation, and treatment goals. GPs should have access to clear contact details for a named specialist or clinical team to address urgent queries, as well as agreed processes for sharing test results or alerts (e.g., mood changes or problematic use). Clinics like Elios Clinics also provide resources such as patient information leaflets, dosing schedules, and guidance on drug interactions, along with follow-up appointments to monitor progress and adjust treatments as needed.
GPs maintain the right to decline prescribing while continuing to support overall care and safety monitoring. If benefits diminish, side effects become problematic, or red flags arise – such as new psychosis, suicidality, cognitive impairment, misuse, pregnancy, or significant cardiorespiratory symptoms – GPs should promptly liaise with the specialist to explore dose adjustments or discontinuation, following best-practice guidelines for unlicensed medicines.
Patient Eligibility and Treatment Management
Which Patients May Be Eligible
Specialist doctors assess patient eligibility on an individual basis, while GPs play a key role in identifying potential candidates and providing support once treatment has stabilised.
Cannabis-based medicinal products are most often considered for conditions like chronic or neuropathic pain, anxiety, PTSD, MS-related spasticity, severe epilepsy, and chemotherapy-induced nausea or vomiting that doesn’t respond to standard treatments. Elios Clinics focuses on treating chronic pain, psychiatric conditions (such as anxiety, stress, depression, and PTSD), neurological disorders, gastrointestinal issues like Crohn’s disease, insomnia, and muscle spasms linked to chronic illnesses.
"Patients must have a confirmed diagnosis unresponsive to conventional treatments." – Elios Clinics
Patients need to have tried at least two conventional treatments at therapeutic doses before being considered. Specialists also review any potential contraindications, such as pregnancy, significant cardiovascular problems, or a history of psychosis. It’s important to set realistic expectations about what medical cannabis can achieve, aligning this process with shared care agreements previously outlined.
NHS prescriptions for cannabis-based treatments are rare and typically limited to specific conditions. In most cases, prescriptions are issued privately through specialist clinics, which may have broader but still rigorous eligibility criteria requiring detailed specialist evaluations.
Assessing Benefits and Risks
Once eligibility is established, the next step is to weigh the potential benefits of treatment against the risks. Specialists initiate treatment, and GPs then continue to evaluate its impact using guidance from the Medical Cannabis Clinicians Society. This process considers past treatment failures and the specific nature of the condition. For example, potential benefits might include reduced pain levels in chronic pain, fewer seizures in epilepsy, or improved muscle spasticity in MS.
At Elios Clinics, specialists conduct thorough assessments – often via video consultations – reviewing the patient’s medical history, symptoms, and previous treatments. Based on this information, they create a tailored treatment plan, specifying the appropriate cannabis strain, THC:CBD ratio, and dosage.
"Specialists offer tailored plans that balance potential benefits with risks." – Elios Clinics
GPs must also carefully evaluate risks, including potential psychoactive effects from THC (noting that some products contain minimal amounts, such as 50µg per unit), dependency concerns, cognitive impairment, and cardiovascular effects. Drug interactions are another critical factor, as cannabis-based products can influence CYP450 enzymes, potentially affecting medications like warfarin, clobazam, or certain antidepressants. Additionally, a patient’s psychiatric history, susceptibility to mood disturbances, and driving-related risks should be reviewed before entering into a shared care arrangement.
To minimise risks, MCCS dosing guidelines recommend starting with low doses – typically 2.5–5mg of THC/CBD oils – and gradually increasing based on the patient’s response. This cautious approach helps identify the optimal dose while reducing adverse effects. Once benefits are established and risks are managed, ongoing monitoring becomes essential.
Monitoring Treatment and Stopping Prescriptions
Patients should be monitored every three to six months using tools like symptom scores, side effect logs, and quality-of-life assessments, which might include patient diaries or validated scales. Relevant tests, such as liver function checks, should also be included. Dosages can be adjusted in consultation with specialists to ensure the treatment remains effective. These monitoring protocols are part of the shared care arrangements and align with specialist guidance.
Elios Clinics provides follow-up appointments to track progress and make any necessary adjustments to treatment plans. They also offer ongoing advice to ensure patients use their medication effectively and recognise any changes in their symptoms.
Treatment should be discontinued if no improvement is observed after three to six months, if adverse effects occur, if the patient becomes non-compliant, or if pregnancy or breastfeeding arises. When stopping treatment, tapering the dosage gradually can help prevent withdrawal symptoms, and the rationale for discontinuation should be clearly documented. In some cases, switching to licensed alternatives like Sativex or Epidyolex might be appropriate.
Certain red flags require immediate specialist input, including the onset of psychosis, suicidal thoughts, significant cognitive impairment, evidence of medication misuse, pregnancy, or severe cardiorespiratory symptoms. While GPs have the discretion to decline prescribing, they remain responsible for supporting the patient’s overall care and ensuring their safety, keeping their wellbeing as the primary concern.
sbb-itb-24979b8
Practical and Legal Considerations
When addressing medical cannabis with patients, GPs must navigate both practical steps and legal responsibilities. Here’s a closer look at how to manage these aspects effectively.
Responding to Patient Requests
Patients should be informed that only GMC-registered specialists are authorised to initiate treatment with cannabis-based medicinal products (CBMPs). If a patient expresses interest, they can be directed to specialist clinics such as Elios Clinics for an eligibility assessment. These clinics allow patients to book consultations directly, without the need for a GP referral. Once a specialist prescribes treatment, the GP’s role involves ongoing monitoring through shared care arrangements.
A supportive way to address patient inquiries could be:
"As your GP, I’m unable to prescribe medical cannabis directly. Only a GMC-registered specialist can initiate this treatment. I recommend contacting a clinic like Elios Clinics for an assessment. If treatment is started, we can work together to monitor your progress safely under a shared care plan."
Documentation Requirements
Accurate and detailed documentation is essential for all consultations and interactions related to CBMPs. This includes recording discussions about risks, benefits, shared care agreements, specialist treatment plans, and any adverse events. All records should comply with GMC standards and guidelines.
For private prescriptions of controlled cannabis-based products, complete the FP10PCD form, ensuring quantities are written in both figures and words. Copies of these forms must be retained for at least two years. Alongside maintaining thorough records, it’s important to remind patients of their legal responsibilities regarding the use of prescribed cannabis products.
Driving, Possession, and Legal Obligations
Patients prescribed products containing THC must be made aware of their legal obligations. They should not drive if cannabis impairs their performance and must notify the DVLA if treatment affects their ability to drive safely. Under strict THC drug-driving laws, patients should refrain from driving if they experience side effects such as drowsiness or reduced concentration.
When it comes to possession, patients are legally allowed to hold cannabis-based products only if they have been dispensed through a valid prescription from a licensed pharmacy. To avoid legal complications, patients should carry their prescription and product labels at all times. It’s also important to note that possession of cannabis without a valid prescription remains illegal. Patients are advised to limit quantities to no more than a three-month supply to avoid suspicion.
For vulnerable patients, such as those with a history of psychiatric issues, safeguarding concerns should be carefully assessed. This includes evaluating risks of dependency or misuse. GPs should document these discussions, advise patients on secure storage of their medication, and schedule regular follow-ups to ensure their care remains safe and well-rounded.
Conclusion
Since 1 November 2018, only GMC-registered specialists have been authorised to initiate CBMPs, while GPs play a crucial role in supporting patients through shared care arrangements once treatment is stabilised. This partnership ensures that patients receive expert oversight from specialists, while GPs maintain continuity of care through regular monitoring and thorough documentation. These roles reflect the collaborative framework previously discussed.
Access to medical cannabis remains highly restricted. NHS prescriptions are limited to a small number of severe conditions, leaving the majority of treatments to the private sector. This underscores the importance of clear communication and coordination between GPs and specialists to navigate these challenges effectively.
Working alongside specialist services like Elios Clinics can significantly improve patient care. These clinics offer eligibility assessments, video consultations, and personalised treatment plans. Their clinicians collaborate closely with GPs, providing ongoing support and addressing any questions throughout the treatment process. This shared care approach aligns seamlessly with the practices highlighted earlier.
FAQs
Are GPs in the UK allowed to prescribe medical cannabis?
No, GPs in the UK are not permitted to prescribe medical cannabis directly. However, they can refer patients to specialist clinics, such as Elios Clinics. At these clinics, GMC-registered doctors carry out thorough assessments to decide whether a patient qualifies for treatment.
These specialist clinics are authorised to prescribe medical cannabis for conditions such as chronic pain, psychiatric issues, or neurological disorders. This approach ensures that treatments remain safe, tailored to the patient’s needs, and fully compliant with UK regulations.
Which conditions qualify for medical cannabis prescriptions on the NHS?
Medical cannabis can be prescribed on the NHS, but only for a small range of severe, treatment-resistant conditions. These include epilepsy, multiple sclerosis (MS), severe nausea and vomiting caused by chemotherapy, and some instances of chronic pain.
To qualify, patients need a confirmed diagnosis of one of these conditions and must have tried conventional treatments without success. Each case is carefully reviewed to ensure that medical cannabis is only considered when all other options have been fully explored.
What is a shared care agreement between GPs and specialists for medical cannabis treatment?
A shared care agreement is a collaborative arrangement between a specialist and a GP, designed to provide safe and effective medical cannabis treatment. The specialist assesses the patient, recommends the appropriate treatment, and outlines a detailed care plan. The GP then steps in to handle ongoing prescriptions, monitor the patient’s progress, and manage their care, all while following the specialist’s guidance.
This teamwork ensures that patients benefit from the expertise of a specialist while continuing to receive consistent care through their GP. It strikes a balance between expert oversight and continuity, prioritising patient safety and well-being.